

Conclusionsīased on the developed model, we are able to propose several design criteria for deconstruction process. We also confirm the well-known importance of cellulose accessibility, which increases with pretreatment. For larger particles ( R > 1000 \(\mu m\)), increases in biomass loading do not offset the significant internal mass transfer limitations, but high enzyme loadings improve enzyme penetration by maintaining a high concentration gradient within the particle. We notably see that increasing biomass loading, while keeping enzyme loading constant should be favored for both small- ( R 1) does not bring a significant benefit. In such cases, diffusion of cellulases to the available cellulose surface limits the rate of glucose release.
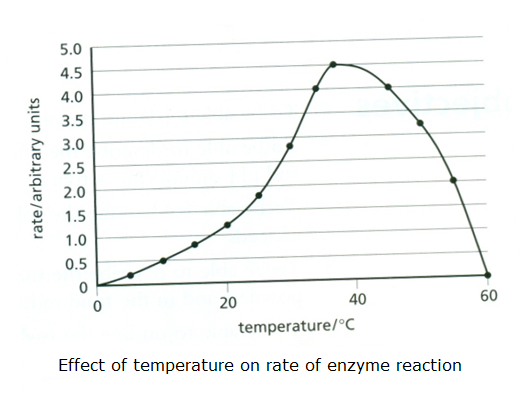
ResultsĮxploring the effect of various experimental and structural parameters highlighted the significant role of internal mass transfer as the substrate size increases and/or the enzyme loading decreases. Our model carefully considers the overall quantity of cellulase present in the hydrolysis mixture and explores its interplay with the available accessible cellulose surface.
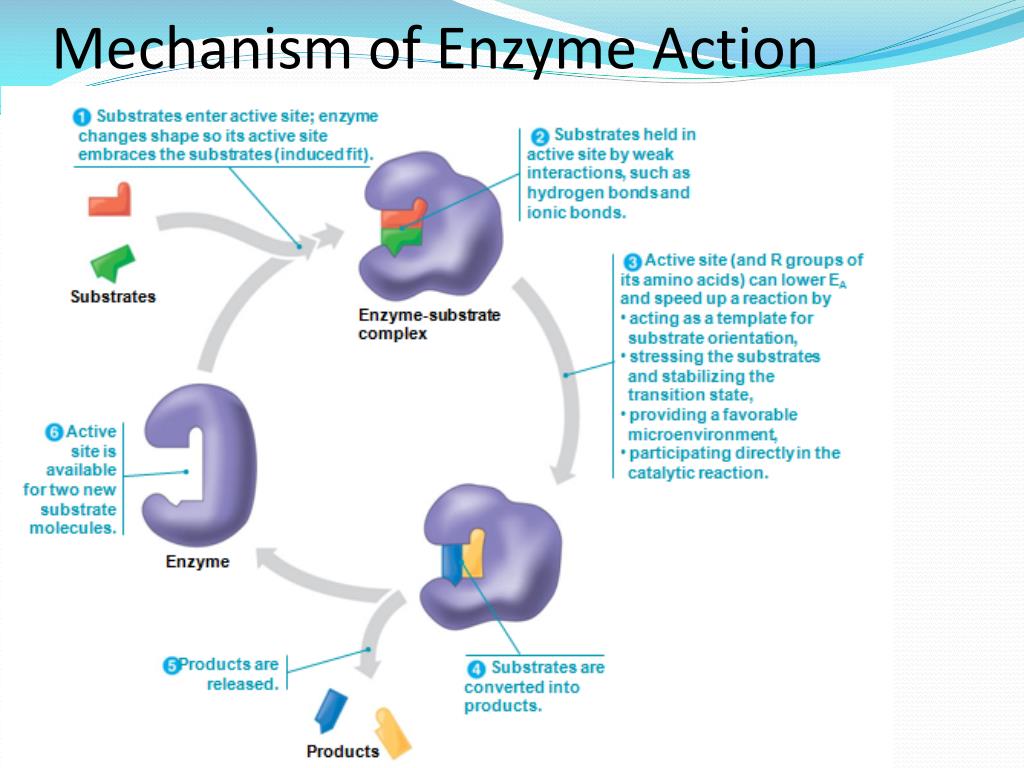
In this study, we used a model based on a set of partial differential equations describing the evolution of the substrate morphology to investigate the interplay between experimental conditions and the physical characteristics of biomass particles as the reaction proceeds. So the enzyme needs to bind the substrate slightly imperfectly in order to be able to turn it over, that is, convert it to the product.Understanding how the digestibility of lignocellulosic biomass is affected by its morphology is essential to design efficient processes for biomass deconstruction. This is the paradox of how enzymes work, they need to be able to bind specifically to the substrate, but they also need to be able to turn the substrate into something else (product), which means those two things are at odds with each other. The Lock and Key model explains that the enzyme needs to bind substrate, but once the reaction progresses to the transition state and product formation, the active site would not be able to accommodate this change. This is because the enzyme needs to bring about a change to the substrate and not just to bind it. The problem with this model is that if the substrate fitted the enzyme perfectly, catalysis would be hampered. There’s some truth in the lock and key model in that enzymes do have active sites, which need to be filled with a substrate and interact with the substrate through non-covalent interactions. Model 1: Lock and Key In this model, the shape of the active site and substrate complement in such a way that the substrate fits into the binding site perfectly.


 0 kommentar(er)
0 kommentar(er)
